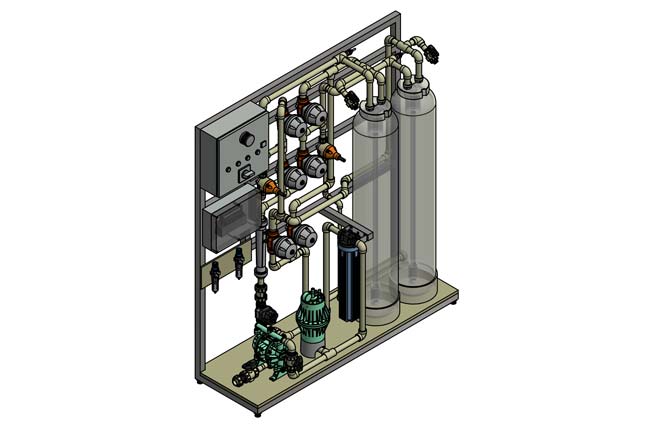
Selective exchanger system, SAT system
A selective exchanger system, SAT system, is used as a so-called police filter in industrial wastewater plants (CP plants, chemical-physical wastewater plants) in the final filtration after precipitation/flocculation or, for example, in groundwater remediation in order to keep regulatory relevant residual heavy metal contents in the wastewater below the required monitoring value.
In industrial chemical-physical wastewater treatment plants, there is usually a batch or continuous treatment of the wastewater produced, in which substances relevant to monitoring are removed from the wastewater by precipitation and flocculation. However, due to the complex composition and discontinuity of industrial wastewater (partial) streams, this treatment alone may not be sufficient to meet the official requirements for wastewater treatment in accordance with the relevant annex of the Wastewater Ordinance or, in the case of indirect dischargers, the drainage statutes of the local municipal wastewater treatment plant. To ensure this in accordance with the state of the art, a selective exchanger system, SAT system, with an IDE exchanger resin (iminodiacetic acid exchanger), e.g. Lewatit TP 207, is used in these cases to remove the heavy metals not sufficiently reduced in the CP treatment, often nickel, copper, zinc or lead, and replace them with Na+, Ca2+ or another cation with which the Ion exchanger was conditioned. Parameters that are not relevant for monitoring, such as alkaline earth metals (e.g. Mg), are not bound by the exchanger to a relevant extent in order not to unnecessarily exhaust the capacity of the exchanger. The order of binding is determined by the ion exchanger selectivity, i.e. the affinity of the individual heavy metals for the iminodiacetic acid group of Lewatit TP207.
The same process can also be used in groundwater remediation, depending on the parameters, possibly also with other resin types than the selective exchanger TP 207, e.g. a weakly or strongly basic anion exchanger for cyanide complexes.
Products
Depending on the design of the system for the customer process, a selective exchanger system has its own regeneration station for on-site regeneration. Due to the often long running times of SE systems, it often makes sense to have the ion exchanger regeneration service carried out externally at the central regeneration station in 92348 Berg.
For a flow rate of between 2.5 and 20 m³/h and for heavily contaminated wastewater, a selective exchanger system with its own regeneration station is advisable. The resins used in the system in the two columns connected in series are regenerated with an acid and activated with an alkali, usually with hydrochloric acid and caustic soda. The regenerates from the plant are then treated in the company’s own chemical and physical wastewater treatment plant. Please note that the system is at least subject to notification under water law.
With a flow rate of up to 5 m³/h, an externally regenerated ion exchanger system is often the more economical solution, in special applications up to 25 m³/h. This means that the ion exchanger cartridges are loaded at the customer’s premises during the customer process and then sent to the central regeneration station in 92348 Berg. After regeneration, the cartridges are returned to the customer. The cartridge size can vary depending on throughput and capacity and is typically between 30 – 1500L resin volume.
Customized adaptation of selective exchanger system:
- Customer-specific design for the wastewater treatment process in existing or new CP wastewater plants (e.g. also wastewater containing Cr(VI) and cyanide) and degree of automation starting from a semi-automated, functional basic design with 2 columns up to an automated and remotely monitored 3-column design.
- Adaptation to any existing control technology and connection to a process control system and the existing structural conditions at the site or the entry to the site
- Preliminary investigations and optional provision of test facilities/technical center possible
- Fulfillment of the legal requirements for divalent heavy metals acc. the annexes to the Wastewater Ordinance acc. the best available techniques (BAT) for IED installations in accordance with of the IE Directive 2010/75/EU or the 4th BImSchV.
- Siemens PLC circuit with/without touch display and with the option of external access
- Duplex version for uninterrupted 24/7 operation as standard, additional protection with a 3rd pillar possible
- Stainless steel or plastic frame construction for corrosive environments
- Pressure tanks in PE/GfK, PVDF/GfK or coated steel
- Selective exchange resin from LANXESS, Lewatit TP 207 or Purolite S930 Plus
- Piping and fittings in PVC, PP, PE or PVDF from the manufacturers GF, GEMÜ (pneumatic or electric) or according to customer requirements; otherwise design with standard industrial components without special elements where possible.
- Temperature design up to 70°C possible, higher temperatures on request. However, please note the maximum soaking temperature of 35°C, which should only be used permanently with special denaturation measures.
- System design for operation in compliance with occupational safety requirements, even in the event of typical misuse
- Disinfection option for the resin bed
- Possibility of pre-acceptance and trial operation in our own workshop
- Modular, maintenance-friendly design according to customer requirements with various optional expansion options, e.g. multi-layer filters, receiver tanks, chemical storage tanks as dosing stations or as AwSV LAU systems, drip pans, simple feeding to redundant FU duplex pressure booster stations, pressure difference display, actual consumption and production data recording, separate resin changeover connections, system access control, valve position feedback, additional filter stages.
- Designed as an ion exchanger to be regenerated externally in a central regeneration station for ion exchangers with exchangeable cartridges in the system (without wastewater generation on site) or as a system with its own automatic regeneration station on site (with wastewater to be treated on site).
We will be happy to help you.